Gait in Parkinson's disease
Decoding neural signatures of gait deficits
MOTIVATION. Impairments of gait and balance are among the most incapacitating and least well-understood symptoms of Parkinson's disease (PD). Patients exhibiting such disorders endure difficulties that severely affect their everyday mobility and independence.
Well-established neuromodulation therapies addressing Basal Ganglia dysfunction in PD, which for decades have been optimized for upper-limb, and are highly effective for the symptomatic treatment of motor signs such as tremor, bradykinesia and rigidity, exhibit much more modest results for gait.
A limitation holding back the design of better therapies is the lack of mechanistic readouts that correlate pathological neural activity patterns and leg dysfunction during gait, which may help characterise and predict when deficits arise, how they change across locomotor activities or under different neuromodulation conditions, and how they may be employed to optimize therapies.
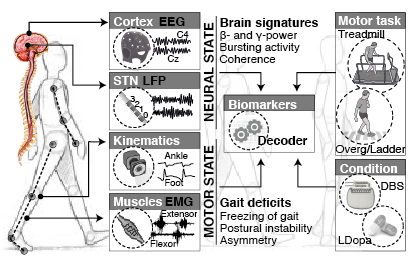
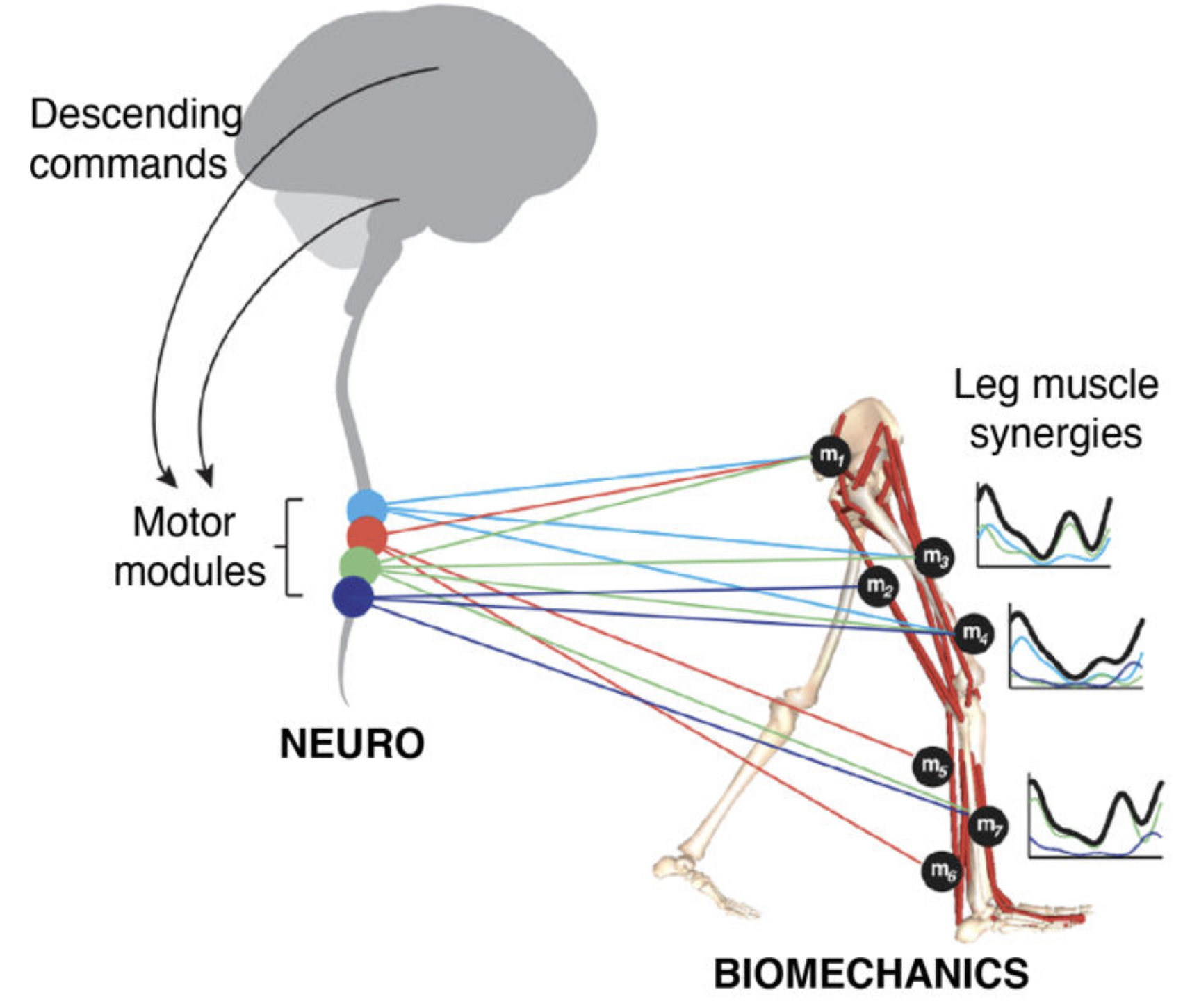
GOAL. We are combining a high-resolution tracking platform established at CHUV, with unique equipment for recording and modulating gait wirelessly and in real-time , in order to map the activity of subthalamic nucleus and cortical networks onto kinematic, kinetic, and muscle activity patterns while patients execute a range of activities of daily living.
Additionally, we are collaborating with a Danish motion-capture company (Rokoko) in the testing and validation of suitable, affordable technologies for whole-body monitoring in clinical applications, that may provide full interaction with patients during unconstrained movements in ecological environments (both inside and outside of the hospital) with fast, easy calibration and complete flexibility.
Our final goal is to link brain commands of motor function and dysfunction to neuromodulation therapies, in order to personalise the delivery of spinal cord stimulation, in combination with deep brain stimulation, and maximise the therautic impact of these treatments in everyday life situations.
FUNDING: European Union H2020 Marie Sklodowska-Curie Action (MSCA-IF- 2017-793419), the Swiss National Science Foun-dation (Ambizione fellowship PZ00P3_180018) and the Parkinson Suisse Foundation
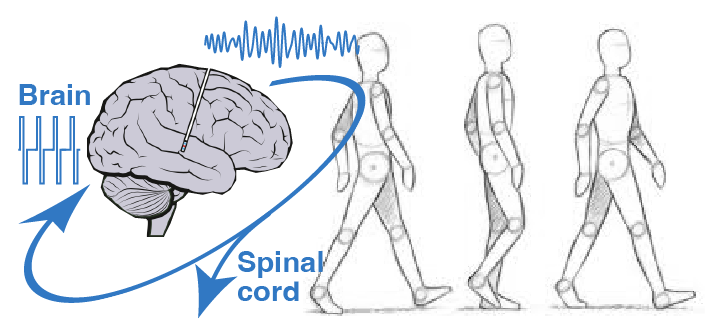
Spinal cord stimulation to alleviate gait and balance deficits
We are exploring the use of spinal cord stimulation to alleviate problems of mobility, improve symmetry, increase walking speed, and reduce freezing of gait.
Decades of research in spinal cord electrophysiology in animal models and humans have shown that epidural electrical stimulation (EES) of the lumbar spinal circuitry enables a selective and well-controlled modulation of the reflex sensorimotor circuits that regulate trunk and leg muscle activation.
In particular, sacral (S1- S2), lumbar (L1–L5) and low thoracic (T12) dorsal roots project to spinal segments containing motor neurons that innervate leg and lower-trunk muscles. EES allows to precisely recruit individual dorsal roots to induce (i) trunk and abdominal muscle contractions, or (ii) hip, knee or ankle flexor and extensor muscle activations.
EES thus has the potential to compensate for pathological descending brain inputs, and facilitate standing and walking in patients with advanced Parkinson's disease, as well with atypical forms of parkinsonianism such a multiple systems atrophy (MSA) or progressive supranuclear palsy (PSP).
Past projects
Linking decision-making and motor pattern generation
in Parkinson's disease
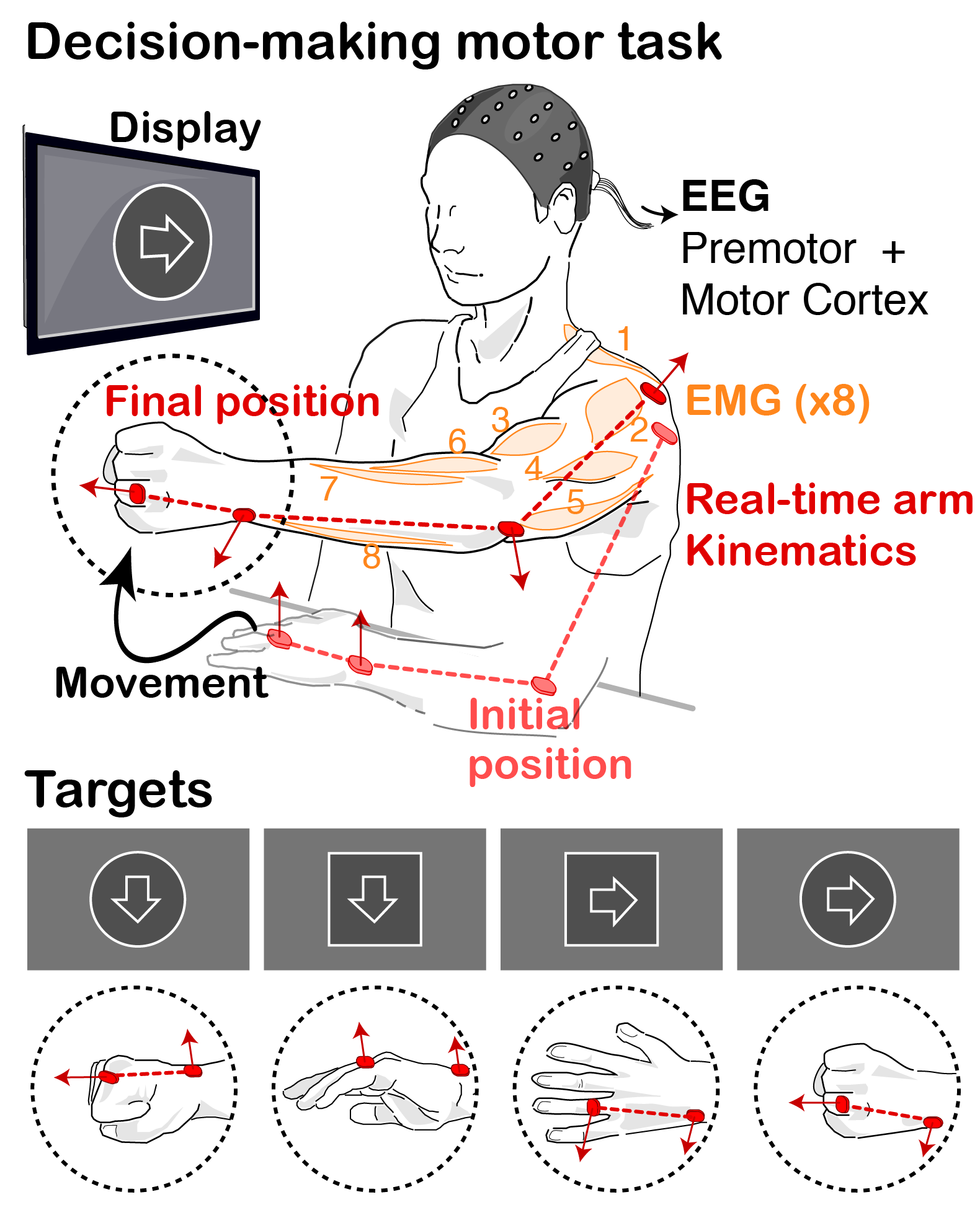
MOTIVATION. Decision-Making studies commonly deal with finite (often binary), well-defined sets of choices. Instead, motor control is traditionnally concerned with continuous, high-dimensional spatiotemporal patterns of kinematic and kinetic changes that give rise to coordinated multi-joint movements. As a result of these dichotomies, the link between these two fields has remained largely unexplored, even though this understanding might be pivotal for the translation of existing theories and models of action selection to the study of motor dysfunction in neurological movement disorders, such as rigidity or bradykinesia.
Over the past decades, theories of motor control have put forward the concept of modularity, whereby motor primitives, or synergies, underlie and help simplify motor pattern generation and coordination. Much work has been devoted to the mathematical procedures and neurobiological plausibility of synergies, along with their use to help asses and guide rehabilitation. Yet little effort has been devoted to understanding how or when these modules are activated by decision making centres during movement.
GOAL. We are combining behavioural experiments and mathematical models to study the interplay between decision making processes and motor pattern generation and adaptation, particularly in the context of Parkinson’s disease.
We hypothesize that the modular organisation of movements might allow to unite theories of action selection and motor control, by providing a finite repertoire of lower-level motor primitives whose activation (or weights) are selectively regulated by decision making centres. In our tasks, we decompose movements into simpler motor components and probe the behavior and performance of patients when constructing, and correcting on the fly , movements requiring flexible combinations of these components. We can then correlate the underlying decisions to the motor states and biomechanical performance, and extract how movements are affected by deficits in specific brain circuits. |
|
CONTRIBUTORS: Dr Pirondini (EPFL), Dr Fisher (Univ Oxford), Dr Britain (Univ Birmigham), Prof Bloch (CHUV-UNIL), Prof Brown and Prof Bogacz (Univ Oxford).
FUNDING: Medical Research Council, UK
Modelling & decoding of pathological brain oscillations
to optimise DBS therapies in Parkinson's Disease
MOTIVATION. Parkinson's disease is characterised by excessive levels of neural synchrony within basal ganglia networks, commonly illustrated by pathological oscillatory brain activity in the beta-band (15-30Hz).
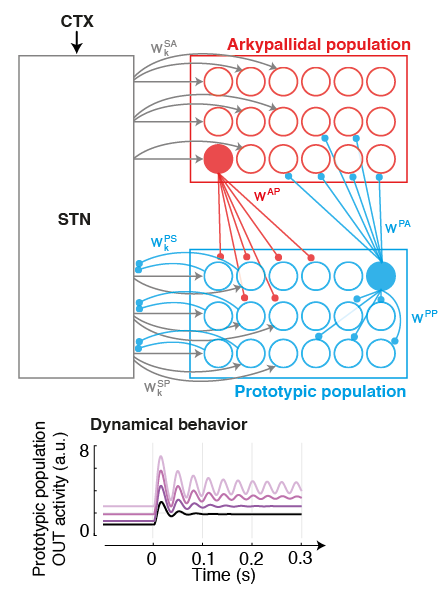 |
This biomarker has been thoroughly studied in past years: It has been identified across structures, and exploited to engineer the first generation of closed-loop Deep Brain Stimulation (DBS) therapies. However the connectivity origins that give rise to such oscillations are still unclear, and the time evolution of neural processes leading up to such synchrony are unknown. GOAL. We are pursuing the development of neural models of key populations in the basal ganglia, together with machine learning-based decoders, in order to understand and predict the origin (and time evolution) of oscillatory patterns. These model-based predictors hold the potential to allow more precise targetting of pathological signatures with DBS, hence reducing side-effects and improving battery life.
|
CONTRIBUTORS: Dr Tinkhauser (Univ Oxford), Prof Brown and Prof Bogacz (Univ Oxford).
FUNDING: Medical Research Council, UK
Closed-loop spinal cord neuromodulation to optimize
motor recovery after spinal cord injury
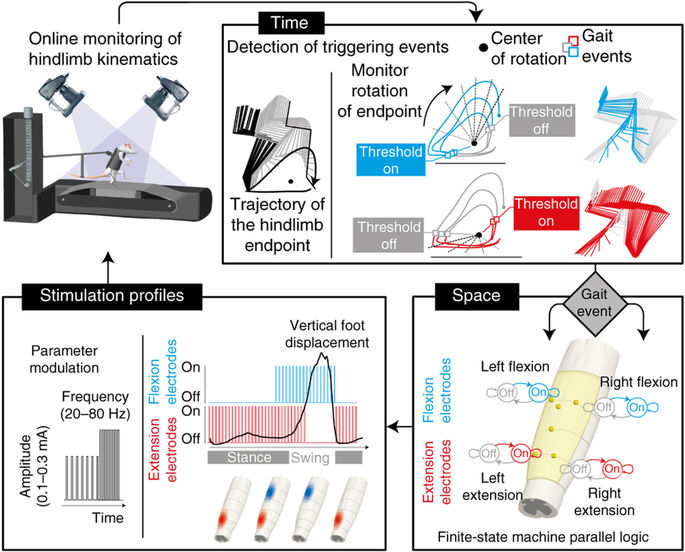
MOTIVATION. Severe lesions of the spinal cord damage the neural pathways that connect sensory feedback circuits in the spinal cord with supra-spinal centres, and interrupt most sources of control and modulation. This loss of connectivity abolishes motor function and leads to paralysis of the limbs. However even in the absence of brain input, pools of motor neurons in the spinal cord are able to exploit specific proprioceptive signals from the legs and to generate coordinated rhythmic movements. After the injury, the SC retains this capacity but lacks the level of excitation required for this control to be manifested.
This neural circuitry may be activated using epidural electrical stimulation (EES) of the spinal cord. EES helps modulate the excitability of these circuits, and restores the capacity of spinal circuits to interpret afferent feedback as a source of control, which can ellicit stepping-like movements in spinal rats on a treadmill. When changing the stimulus parameters, we can interact with the ongoing spinal dynamics in real-time and tune the level of exitability, which in turn regulate motor output. However, there are few guidelines available to guide the selection of the appropriate stimulus parameters; from an experimental point of view, their tuning is largely an ad hoc manual process that requires of extensive human expertise in order to comprehend the highly complex (and often unknown) dynamics of gait induced by EES.
GOAL. We are pursuing the development of automatic control approaches to optimize the stimulation paradigm online in space and time, accounting for the specific biomechanical state of the system in order to induce more natural and repeatable movements that may lead to improved rehabilitation. |
|
CONTRIBUTORS: Dr Capogrosso (Uni Fribourg), Prof Courtine (EPFL), Prof Bloch (CHUV-UNIL).
FUNDING: EU FP7 program (project NEUWalk)
Credits: EPFL |
Eduardo Martin Moraud 2020. 3D designs and photography by Jimmy Ravier. All rights reserved